Transition rapidly to final product validation
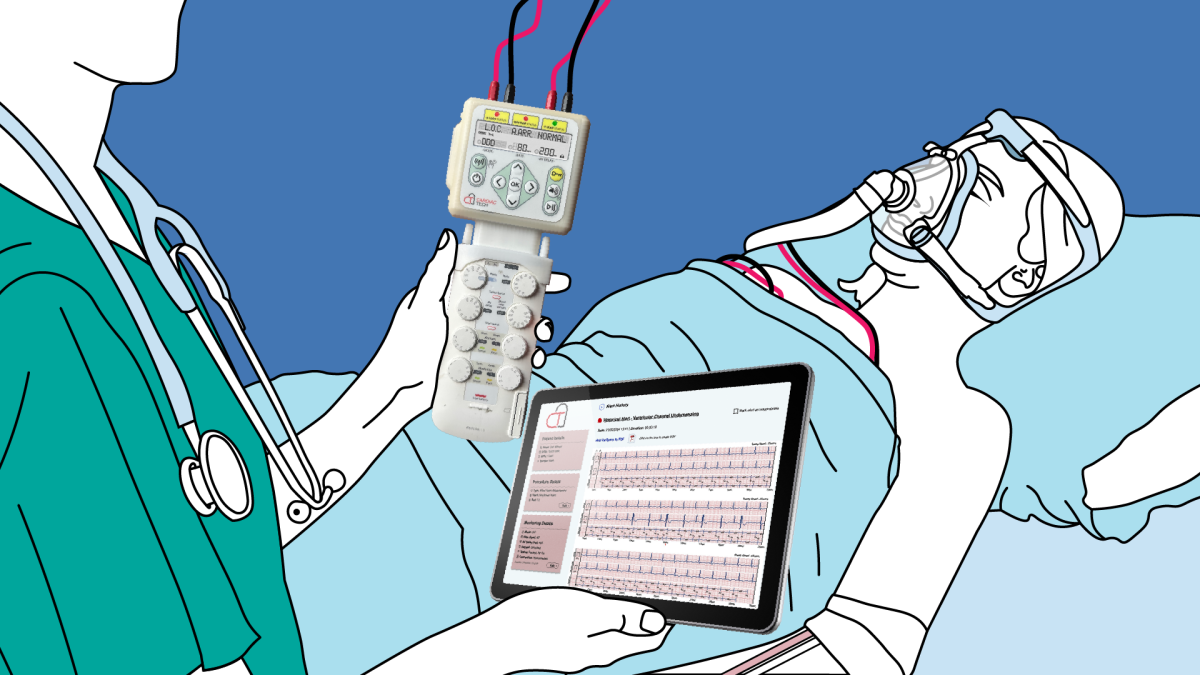
The last step before submitting a hardware or software medical device for certification is collecting validation evidence to show that the device fulfils its sales claims.
This can only be done with the final version of the device that has been built to full regulatory standards and is hosted with Service Level Agreements that meet the local regulatory and legal requirements for data privacy.
At Camgenium, transitioning from the Prototype stage to the full production prototype is rapid and straight forward.
- Development work has met regulatory standards
- Your technical file has been kept up to date throughout development
- We can rapidly transition hosting to required SLAs
- We are expert at productionising, for example, we are highly experienced at submitting medical apps to be scrutinised by Apple and Google for mounting on the AppStore and Play Store

Developing quickly without adhering to standards is almost always a false economy as these steps usually have to be redone.
Dr Philip Gaffney OBE
CEO, Camgenium
Read more about how we make transitioning to production prototype easy.